
Fluorescent in situ hybridization as a genetic technology to analyzing chromosomal organization of alien wheat recombinant lines.

Schwarzacher T, Ali N, Chaudhary HK, Graybosch R, Kapalande HV, Kinski E, Heslop-Harrison JS. In the future this homologous area in A genome could be substituted with radish chromosome area carrying the Rfk1 gene.Īvailable from Physical-Mapping-Technologies ISBN 978-92-0-119610-1Ģ94. rapa A genome was verified with BLAST analysis. Using the BAC-FISH analysis, weak signals were sometimes visible in two chromosomes of turnip rape and a homologous region of Rfk1 in chromosome 9 of the B. Detected disomic addition lines were found to be unstable for turnip rape hybrid production. In the BAC-FISH analysis, double dot signals were detected in sub-terminal parts of the radish chromosome arms showing that the fertility restorer gene Rfk1 was located in this additional radish chromosome. The GISH analyses clearly showed that the turnip rape restorer plants were either monosomic (2n=2x=20+1R) or disomic (2n=2x=20+2R) addition lines with one or two copies of a single alien chromosome region originating from radish. Both probes showed a signal in the chromosome spreads of the restorer line 4021-2 Rfk of turnip rape but not in the negative control line 4021B. The metaphase chromosomes were hybridized using radish DNA as the genomic probe and BAC64 probe, which is linked with Rfo gene. The physical localization of the radish chromosomal region carrying the Rfk1 gene was investigated using 8 GISH (genomic in situ hybridization) and BAC-FISH (bacterial artificial chromosome fluorescence in situ hybridization) methods. The trait was, however, unstable in subsequent generations. A Kosena fertility restorer gene Rfk1, homologue of the Ogura restorer gene Rfo, was successfully transferred from oilseed rape into turnip rape and that restored the fertility in female lines carrying Ogura cms. oleifera) the most promising F1 hybrid system would be the Ogu-INRA CMS/Rf system. Author self-archive preprint linked here. Size and location of radish 1 chromosome regions carrying the fertility restorer Rfk1 gene in spring turnip rape. Niemelä T, Seppänen M, Badakshi F, Rokka VM, Heslop-Harrison JS. Location of the fertility restoration gene Rfk on an unstable radish chromosome in turnip BrassicaĢ95. The understanding of genome evolution will be of critical value both for conservation of the biodiversity of the plant kingdom and addressing the challenges of breeding new and more sustainable crops to feed the human population. DNA sequence information, combined with appropriate informatic tools and experimental approaches including generation of synthetic hybrids, comparison of genotypes across environments, and modelling of genomic responses, is now letting us link genome behaviour with its consequences. We now understand many of the genome-scale processes occurring during evolution involving mutations, amplification, loss or homogenization of sequences rearrangement, fusion and fission of chromosomes and horizontal transfer of genes or genomes through polyploidy or other mechanisms. Understanding genome evolution is critical for biodiversity conservation and breeding sustainable cropsĭarwin recognized the processes of speciation and the extinctions of species. Sequences, synthetic hybrids, comparative genomics and modelling link genome behaviour and consequences Evolutionary processes may be continuous or episodic and have contrasting long and short-term consequences Repeated sequences, not genes, can be localized or dispersed in the genome and make up most of the DNA Genome-scale evolution involves mutation, chromosomal rearrangements, hybridization and polyploidy Genome evolution: extinction, continuation or explosion? Current Opinion in Plant Biology 15:115–121. How do genomes evolve and what is the future for a genome?Ģ96.
#Iaea tecdoc 1188 download#
Link to PubMed database and paper downloads (in Pubmed, click on the 'page' icon on the left hand side to download article).
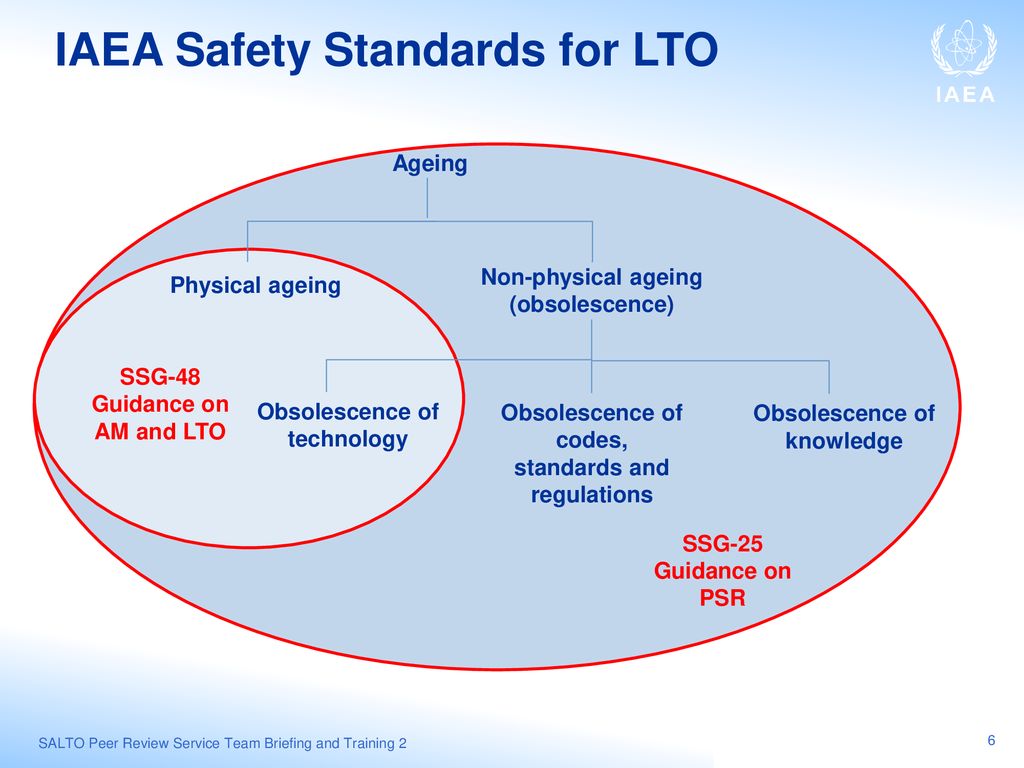
(Includes this complete file under publications side-bar) (Pat) Heslop-Harrison, Department of Biology, University of Leicester, Leicester LE1 7RH, UK.
#Iaea tecdoc 1188 full#
(Links to full text via titles mostly only work for Leicester Intranet others please e-mail me for reprints or use DOI links via publisher's websites or Pubmed some are available on the Leicester Research Archive) Publication List with Abstracts of papers since c. Molecular Cytogenetics and Plant Cell Biology Group


 0 kommentar(er)
0 kommentar(er)
